General context
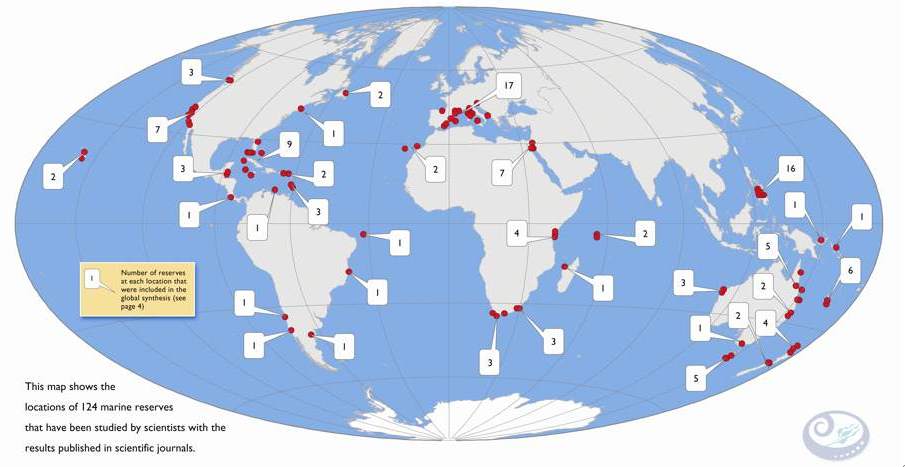
Fishing is commonly considered among the greatest threats to marine ecosystem health. Fish stocks, as any other living resource, cannot be exploited beyond their capacity of renewal, which is assured by the periodic arrival of juvenile fish. If fishing is too intense, fish stocks may dramatically decline. From this perspective it emerges the need for proper ecosystem conservation and fishery management in order to maintain ecosystem health and related services provided to humans (e.g. the persistence of fishing resources). Marine protected areas (hereafter MPAs) have gained popularity on a global scale (Fig. 1) as management options for marine conservation and fisheries management. Many scientific studies stressed the success or failure of single MPAs, especially in terms of recovery of fish populations within their boundaries.

Fig. 1 – Map of 124 marine protected areas (named marine reserves) world-wide (From www.piscoweb.org).
Recovery within MPAs is the prerequisite to then expect other effects of protection, like the export of adult and juvenile fish (i.e. spillover) and/or eggs and larvae, with possible benefits outside MPAs. The positive effects of protection within and in the areas adjacent to MPAs are thought to be powered by the creation of networks of MPAs, i.e. systems of ecologically connected MPAs. This is attributable to the fact that MPAs making part of a network may 1) reciprocally act as sources and/or sinks of individuals (both adult and juvenile stages), which may make them more resistant against various human impacts, and 2) produce more evident benefits also in areas outside the MPAs. Designing MPAs (e.g. in terms of shape and size) or networks of multiple MPAs (e.g. distance at which MPAs should be created; Fig. 2) is not at all a simple task. The ‘connectivity', i.e. the level of exchange of individuals (larvae, juveniles and adults) among local populations, is the crucial process that could explain, for instance: 1) whether fish population persistence within a MPA depends on larvae/juveniles produced by local spawners living within the MPA (i.e. local retention of larvae/juveniles) or on larvae/juveniles coming from other (fished) areas; 2) whether and how (i.e. at which spatial scale or distance) the MPAs contribute to fish population persistence outside; 3) whether adult fish move across the MPA borders; 4) how the existing MPAs can be re-designed to be scaled on larval/juvenile dispersal, or on adult fish movement patterns; 5) how multiple MPAs can influence each other and surrounding (fished) areas.
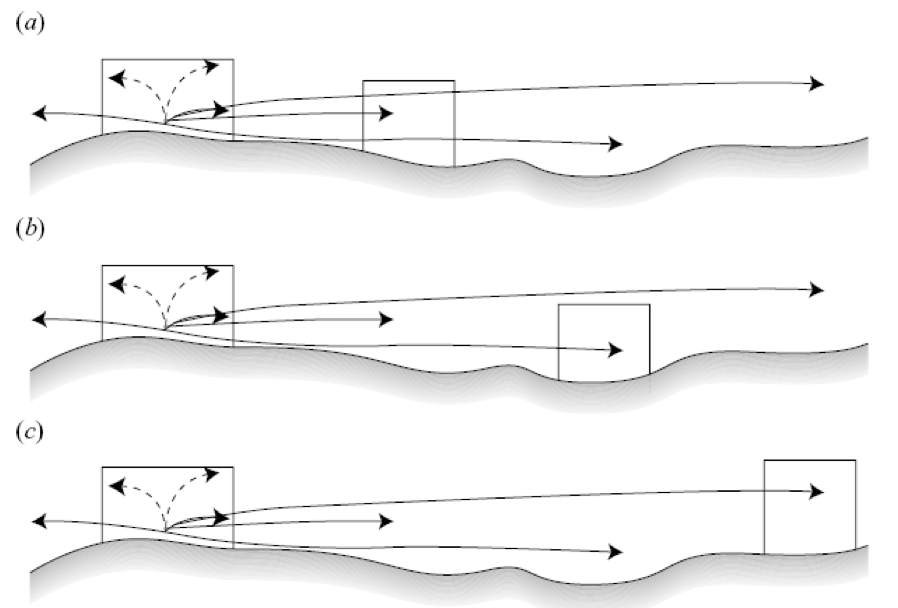
Fig. 2 – Possible reserve spacing within a network given a relatively sedentary species with an extended larval phase. Three possible reserve spacing options along a hypothetical coastline are presented assuming that large fishes (dashed arrows) disperse relatively short distances and larvae (solid arrows) can disperse varying distances for the stock area of a species (From Halpern and Warner 2003).
Information and data about the above points are essential to design networks of multiple MPAs. In other regions of the world, such issues have been investigated by using a wide array of approaches, from modeling to field observations and laboratory experiments. In the Mediterranean Sea, which is one of the regions where it has been invested most in establishing MPAs, however, such studies are scanty and much more is yet to be done. This means that most single already established MPAs have been designed without any basic information to fulfill conservation and management needs at local scale (i.e. conservation within the boundaries and local benefits to adjacent areas) or at large scale (i.e. in the perspective of creating effective MPA networks).
The study area: the MPA of Torre Guaceto
The locality called Torre Guaceto (Fig. 3), situated in the SE Italy (Adriatic Sea), hosts an effective MPA where the surveillance is active and permanent. The whole MPA covers about 2220 ha and develops along about 8 km of coastline.

Fig. 3 – Aerial view of the Torre Guaceto tower, situated in front of the no-take/no-access zone of the Torre Guaceto Marine Protected Area
According to the Italian law, the MPA is divided in three zones:
Zone A (or integral reserve, where the access is permitted only for surveillance and research).
Zone B or general reserve (where only access and swimming are allowed during daytime);
Zone C (partial reserve, i.e. a buffer zone where artisanal fishery is allowed for a limited number of fishermen and under a strict regulation).
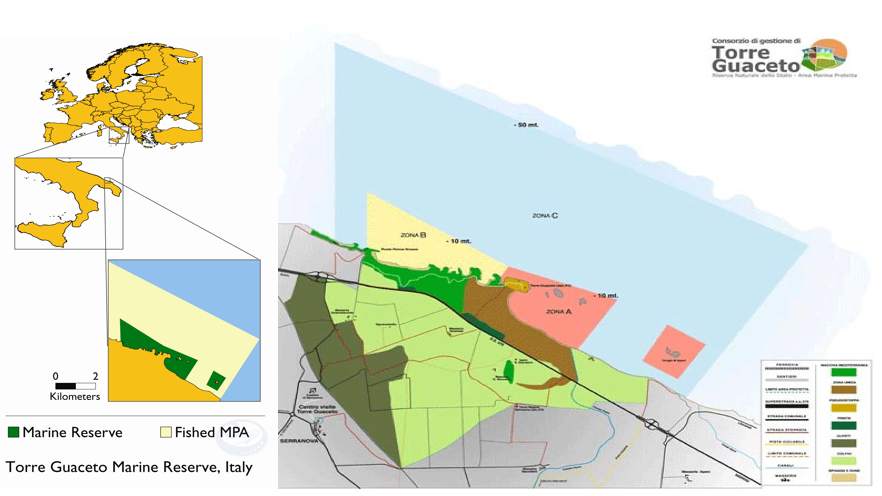
Fig. 4 – Maps of the Torre Guaceto MPA. Left: geographical location of the MPA with the indication of the area open to fishing. Right: subdivision of the MPA into zones A, B and C.
Right: subdivision of the MPA into zones A, B and C.
The MPA was established in the 4 of December 1991, but effective enforcement began around 2001. The Management Consortium of Torre Guaceto was developed at the end of the year 2000. The Consortium is formed by the Town Councils of Brindisi and Carovigno and the WWF Italy, the largest Italian environmental organization. The Torre Guaceto MPA has a high variety of biodiversity (Fig. 5).






Recent investigations demonstrated that effective protection at the MPA allowed both recovery of fish and benthos within its boundaries. Benefits are also produced for local fisheries (Fig. 6).

Fig. 6 - Fishermen in action after having operated in the buffer zone of the Torre Guaceto MPA (Photo D. Fiorentino).
Studied species
The species Diplodus vulgaris (known with the common name of “two banded sea bream”; Fig. 7) is an ecologically relevant species in Mediterranean rocky reefs. The two banded sea bream is one of the most effective fish predator of sea urchins (together with white sea bream and some labrids), and effectively contributes to control sea urchin population density.

Fig. 7 – Adults specimens of the two banded sea bream (Photo E. Trainito).
When abundant, sea urchins overgraze erected macroalgae and mostly remove algal cover from rocky reefs over wide areas. From this perspective Mediterranean rocky reefs may undergo transition from macroalgal-dominated reefs to coralline barrens (i.e. rocky deserts) if fish predators of sea urchins (e.g. two banded sea bream) are overfished. When released from the predation control, in fact, sea urchins may increase in density and overgraze erected macroalgae (Fig. 8). This transition involves dramatic alterations in ecosystem functioning: e.g. reefs deprived of macroalgae are less productive and less effective as nurseries for juvenile fish.

Fig. 8 – Two opposite state of Mediterranean rocky reefs: macroalgal forest (on the left) and sea urchins barren (on the right) (Photos P. Guidetti).
The two banded sea bream is an economically important species being targeted by both commercial and recreational fisheries. It is distributed along Mediterranean coasts, the Atlantic Ocean and from the Gulf of Guascogna to Senegal. It lives on rocky, sandy bottoms and close to Posidonia oceanica beds. Very few information is available so far about behaviour of this fish but, by means of the scarce findings available, adults seems to be relatively sedentary and demersal. This species becomes mature at about 18 cm, during its second year of life, and the spawning period occurs from October till January. Spawners produce eggs and larvae that develop in the pelagic waters before post-larvae metamorphose and settle in shallow coastal benthic habitats. The juveniles of D. vulgaris settle in very shallow water (less than 2 meters). They settle preferentially in sheltered bays (Fig. 9), on rocks and pebbles covered by macroalgae, often intermixed with sand patches, where they can be found as single individuals or in schools. It is known that juveniles of D. vulgaris settle (when they are about 1 cm long) in two pulses: in November-December and in January-February. Some months later (i.e. 6) they move to deeper waters and join the adult population (this phase is named recruitment).

Fig. 9 – Bay inside Torre Guaceto Marine Protected Area where post-larvae of two banded sea bream settle (Photo P. Guidetti).
Although it is one of the most frequent and abundant sparid fish in the coastal waters of southern Europe, the amount of information available on several important aspects of its biology and on their stock assessment is very scarce.
Aim of the project
The present project funded by Total Foundation is aimed at: 1) investigating population connectivity and the MPA effectiveness; 2) providing indications for establishing effective networks of Mediterranean MPAs in terms of conservation of key-species for the overall rocky-reef ecosystem health. Specific goals will be to gather an extensive data set on sea bream populations within and outside the MPA, including (a) abundance patterns of adults and juveniles; age and size distribution; (b) sex ratio and fecundity; (c) natal origin, dispersal of egg and larvae and pelagic larval duration; (d) movement patterns of post-settlers and adults; (e) fish population genetics and connectivity. In order to achieve the goals cited above, the research program is being developed through a tight integration of field work (Fig. 10) and laboratory analyses.

Fig. 10 – Examples of underwater visual census (UVC) of adult (sx: photo E. Trainito) and juvenile fish (dx: photo P. Guidetti).
a) Abundance, size and age distributions of the white sea bream (from juveniles to adults) within and outside the MPA Sampling is aimed to estimate densities and sizes of settlers, recruits and adults. Sea bream density and size distribution are estimated according to standardized procedures directly in the field (i.e. underwater) at 1 location within the MPA, and 6 locations outside (three northwards and three southwards, along the Adriatic Apulian coast, until about 100 km from TGMPA borders). Each location is defined as a stretch of coastline about 30 km long and encompassing 2 randomly selected sites (up to 8 km far from each other). At each site 8 replicated visual censuses are carried out for a total of 112 replicates per each life stage. In addition, we are collecting sea bream otoliths to build up a relationship between age (estimated from otoliths; Fig. 11) and individual size (e.g. measured on collected specimens). This relationship will allow us to construct age-frequency distributions in protected and unprotected conditions, which will be useful to better understand how many years of protection are necessary for a complete population recovery. At this stage a number of >400 individuals, from juveniles to large sized adult specimens, have been collected in the field and (more often) bought from fishermen.
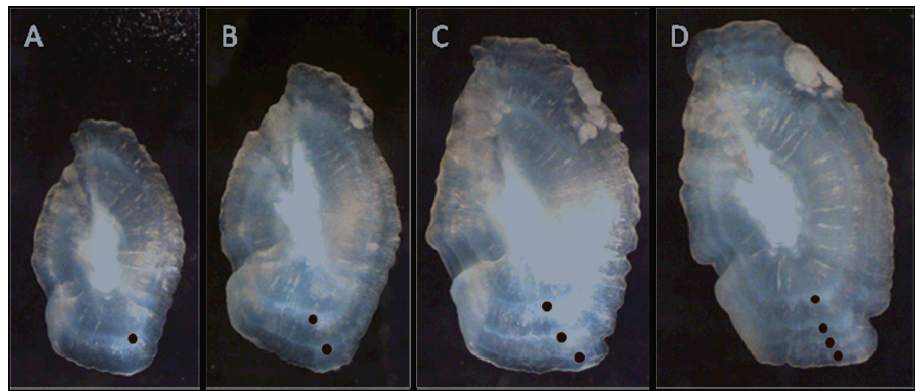
Fig. 11 – Examples of Diplodus vulgaris otoliths taken from fish of different ages. Red dots indicate translucent (or hyaline) rings (reflected light) (Photo P. Guidetti).
b) Sex ratio and fecundity of the white sea bream in relation to individual size and age We are conducing sampling of specimens within a wide range of individual size and are investigating the reproductive biology (e.g. gonadosomatic index, size/age at first maturity; Fig. 12), sex ratio and fecundity of the white sea bream (according to standardised approaches) in order to quantify the increase of the reproductive potential in relation to fish size. At this stage more than 100 specimens, collected both inside and outside Torre Guaceto Marine protected area, have been analyzed.
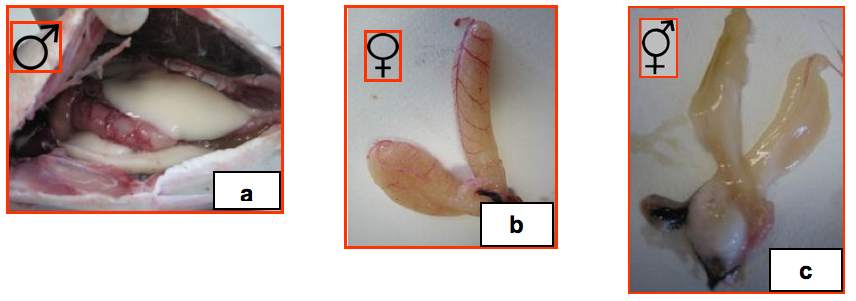
Fig. 12 – Gonads of two banded sea bream (a: testis; b: ovaries; c: hermaphrodite with intersexual gonad)(Photo R. Sahyoun).
c) Natal origin and pelagic larval duration of white sea breams settled within and outside the MPA No information is available on the parental origin of local populations or on the natal origin of recently arrived settlers of the white sea bream. These pieces of information are, however, extremely important for conservation and sustainable fishery management. Larval/juvenile supply in protected or fished populations, for instance, could depend on self-supply or supply from other populations (protected or not) elsewhere, according to a source-sink model. Conservation or fishery management measures strictly depend on this. In this perspective we are investigating, by sampling juvenile white sea breams within and, at increasing distance, outside the MPA, and by conducting otolith chemistry analyses with LA-ICPMS (Laser Ablation Inductively Coupled Plasma Mass Spectrometry) the homogeneity/heterogeneity of natal origin and connectivity among protected and unprotected fish populations. There are no precedents of these investigations in the Mediterranean Sea. This approach may provide information on early life-history dispersal. In addition, the same otolith samples will be treated and analysed at the microscope to determine the pelagic larval duration (PLD), i.e. the number of days spent in open waters before settlement (Fig. 13). At this stage 140 settlers were sampled from 14 sites and all the otoliths were extracted.

Fig. 13 – An example of otolith from settler of two banded sea bream treated to estimate PLD. Each ring corresponds to a day of life of the larva/fish (Photo A. Di Franco).
d) MPA and outside areas as nurseries for the white sea bream Mortality rates during the early phases of the life history may affect the number of juveniles joining the adult population. Recruitment is one of the crucial phases that guarantees the population renewal, persistence and/or recovery from impacts. The nursery role of habitats is therefore crucial to sustain local fish populations. However, there are no evidences in the Mediterranean Sea about the juvenile post-settlement origin (different from the natal origin) of fish that, in the adult phase, can live at locations situated within and outside MPAs. To answer the question about whether or not adult white sea breams living in the studied MPA (or those outside) settle and spend the first months of life within or outside the MPA, we will sample post-recruits/subadults in the MPA and outside at different distances, and we will extract otoliths. The LA-ICPMS will be used for chemical analyses of the juvenile portion of the otoliths. Chemical composition of otoliths (related to trace elements present in the water where they stay) will clarify the degree of homogeneity/heterogeneity of nursery sites for fish that coexist in the adult phase at the sampling locations within or outside the MPA. In other regions of the world this approach has been used to obtain important information for conservation and management of fish stocks in coastal areas. e) Fish population genetics and connectivity In May and June 2010, 755 juveniles of Diplodus vulgaris were collected using hand nets from 7 locations: one in the MPA of Torre Guaceto (SW Adriatic, Italy) and six outside the MPA (three northwards and three southwards), at distances of about 5, 20 and 100 km from the MPA borders. Each location was composed of two sites in which more than 50 juvenile specimens were collected and preserved in 95% ethanol. At this stage 343 adult specimens of Diplodus vulgaris were collected out of 700 scheduled. Adult specimens were collected using longlines, by scuba diving or bought from fishermen. Adults were fin clipped and preserved in 95% ethanol. To investigate the scale of connectivity between the MPA of Torre Guaceto and the surrounding areas, the assignment test will be used. In assignment methods, an individual is assigned to the most likely source population, based on the expected frequency of its multilocus genotype in various putative sources. This method can be applied using hypervariable molecular markers such as microsatellites. They are nuclear repeat unit of 2-3 bp and can get dozens of loci relatively easily. They are also highly polymorphic, neutral, codominant, very abundant and frequently used in genetic studies to detect population structure.
Fig. 14 – Laboratory analyses on DNA of settlers of D. vulgaris
Scientific publications (where Total foundation was acknowledged):
- Di Franco A., Guidetti P., in press. Patterns of variability in early-life traits of fishes depend on spatial scale of analysis. Biology Letters doi: 10.1098/rsbl.2010.1149.
- Di Franco A., De Benedetto G., De Rinaldis G., Raventos N., Sahyoun R., Guidetti P., in press. Large scale variability in otolith microstructure and microchemistry: the case study of Diplodus sargus sargus (Sparidae) in the Mediterranean Sea. Italian Journal of Zoology doi: 10. 1080/11250003.2011.566227.
Outreach and dissemination activities
- Project page in the CoNISMa website: http://www.conisma.it/total/total.html
- Link in the Torre Guaceto MPA webpage: www.riservaditorreguaceto.it
- Facebook link: www.facebook.com/Riserva.TorreGuaceto/posts/200204340018093
- Scientific divulgative article in Italian to be published in the magazine ‘Gazzetta Ambiente’.
- Presentation of the project on the Bulletin of the Italian Society of Marine Biology (2011).
- Public presentation of the project activities to a Croatian delegation (MPA managers, fishermen, policy makers, researchers) at the Torre Guaceto MPA on 8/3/2011.